Steel Elements
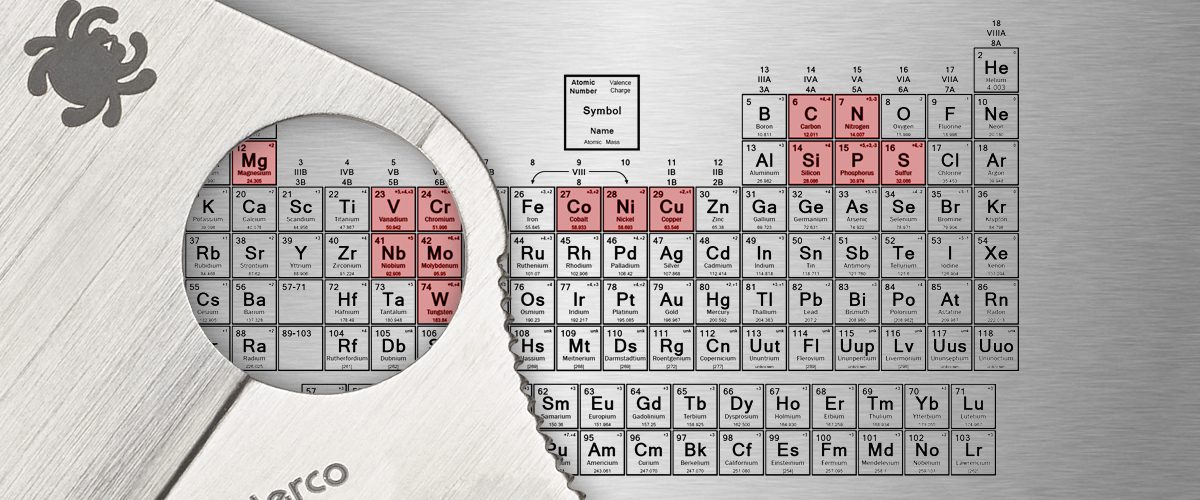
- Carbon (C)
- • Increases edge retention and raises tensile strength.
• Increases hardness and improves resistance to wear and abrasion. - Chromium (Cr)
- • Increases hardness, tensile strength, and toughness.
• Provides resistance to wear and corrosion. - Cobalt (Co)
- • Increases strength and hardness, and permits quenching in higher temperatures.
• Intensifies the individual effects of other elements in more complex steels. - Copper (Cu)
- • Increases corrosion resistance.
- Manganese (Mn)
- • Increases hardenability, wear resistance, and tensile strength.
• Deoxidizes and degasifies to remove oxygen from molten metal.
• In larger quantities, increases hardness and brittleness. - Molybdenum (Mo)
- • Increases strength, hardness, hardenability, and toughness.
• Improves machinability and resistance to corrosion. - Nickel (Ni)
- • Adds strength and toughness.
Niobium (Nb)
• aka columbium. Improves strength and toughness.
• Provides corrosion resistance.
• Improves grain refinement and precipitation hardening
Nitrogen (N)
• Used in place of carbon for the steel matrix. The Nitrogen atom will function in a similar manner to the carbon atom but offers unusual advantages in corrosion resistance.
Phosphorus (P)
• Improves strength, machinability, and hardness.
• Creates brittleness in high concentrations.
Silicon (Si)
• Increases strength.
• Deoxidizes and degasifies to remove oxygen from molten metal.
Sulfur (S)
• Improves machinability when added in minute quantities.
Tungsten (W)
• Adds strength, toughness, and improves hardenability.
Vanadium (V)
• Increases strength, wear resistance, and increases toughness.
Steel Production
The world of steel is as fluid as molten metal. It is ever-evolving. Steel as a matter of opinion is very subjective as it relates to knives and knife knuts. There is no clear-cut answer as to which is the best steel. We have different requirements and preferences.
Our hope is this guide will help you understand the world of steel a bit better and perhaps assist you in better defining what your own preferences are and why. A word of caution, this information is not intended to be all-inclusive, nor could it ever be.
We at Spyderco, just like all other people, gravitate towards superior products. We are committed to using the best materials available at the time. As the world of steel evolves, so do our products. There are over 3000 different types of steel, each having its own positive and negative attributes. In order to determine your own preferences, it is perhaps best to first understand the history of steel and how it is made.
Although an exact date of discovery is not known, man has been forging steel for as long as he's been working iron. Ironworkers learned to make steel by heating wrought iron and charcoal (a source of carbon) in clay boxes for a period of several days. By this process, the iron absorbed enough carbon to become a true steel.
Iron by itself is a relatively soft metal, it does not hold a good edge. However, if you add Carbon it hardens the iron, making steel. Steel has proven to be ideal for making edged weapons.
At a very simplified level, making steel is like baking a cake. You follow a precise recipe to achieve the type of cake (steel) that you desire. You begin with flour (iron) and from there you add various ingredients (elements). These additional ingredients will determine what type of cake (steel) you end up with. Once you have added all of the additional ingredients (elements) you are left with a batter that is ready to bake (heat treat). Baking (heat treating) is just as much a part of the "recipe" as the ingredients (elements). If not done properly, several properties can suffer. Once baked, you have a new – completely different – finished product. Your cake will forever be a cake, it can never go back to being batter. Of course, steel can be re-melted to a molten state, but that simply is the beginning of becoming a new type of steel.
Steel is an alloy of iron and carbon; just as bronze is an alloy of copper and tin. Historically, steels have been prepared by mixing the molten materials. Alloying elements are melted and dissolved into molten iron to make a steel. The molten steel is cast into an ingot, which is then rolled out (while it is still hot) and shaped much like you would roll out cookie dough. As the steel begins to slowly cool below the critical temperature, things start to happen inside the steel. At these elevated temperatures, alloying elements are able to move around in the steel, or diffuse. Different elements diffuse at different rates, (typically the larger the atom, the slower it diffuses). If the alloying contents are too high for some elements to assimilate with, the excess will separate or segregate out of the steel and form inclusions or possibly combine with another element to form large undesirable carbides. These diffusional processes are also controlled by the austenite grain size of the steel – grains are little packets of specifically oriented crystals. Grain boundaries act as barriers to diffusion, the smaller the grains, the more boundaries, and the slower the steel. This limits the performance capabilities of the steel both in corrosion resistance and in wear resistant carbide formation.
More recently, Powder Metallurgy has become the chosen method of preparation. The difference in the processing of a powdered metal allows for steel chemistries not possible with traditional steelmaking practices. The process starts out the same as wrought steels – alloying elements are added and dissolved into molten iron. Then comes the main difference. The molten steel is atomized (misted into microscopic droplets) into liquid nitrogen where the steel is instantly frozen, leaving no time for diffusional processes. The chemistry of the resulting powder is identical to that in the molten vat. Additionally, there are no inclusions or large carbides that form. The austenite grain size is the size of the powder at the very largest, which is small. The powder is then cleaned and sorted by size and then the remaining ideal powder is sintered in a hot isostatic press to solidify the steel. Sintering is heating the steel to a temperature just below its melting point, and then pressing it together at high pressures to solidify or remove the voids between powder spheres. This allows for drastic changes in the steel chemistry namely in carbon and vanadium. A larger volume of the highly wear resistant vanadium carbides form upon heat-treating. Since Vanadium has a greater propensity to interact with carbon and form carbides than it does with Chromium, most of the excess carbon is utilized in the formation of vanadium carbides. These leave the Chromium free to help keep the steel corrosion resistant. The result is a premium steel product with properties of exceptional wear-resistance and good corrosion-resistance.
Heat treating the steel to its critical temperature allows the carbon atoms to enter into the crystalline molecules of the iron which have expanded due to the heating. Quenching the steel at this point causes the molecules to contract, trapping the carbon atoms inside. More specifically, the process of hardening steel by heat treatment consists of heating steel to a temperature at which austenite is formed. Austenite has the property of dissolving all the free carbon present in the steel. Quenching is then used to "freeze" the austenite changing it to martensite. These treatments set up large internal strains in the steel; these are relieved by tempering (further heating the steel at lower temperatures). Tempering the steel decreases the hardness, strength, and brittleness. It, however, increases the ductility and toughness.
Steels are classified accordingly with the elements used in production. These classifications are Carbon Steels, Alloy Steels, High-Strength Low-Alloy Steels, Stainless Steels, Tool Steels and Exotic Steels (non-steel).
Properties of Steel
- Alloy
- A material that is dissolved in another metal in a solid solution; a material that results when two or more elements combine in a solid solution.
- Alloy Steels
- Have a specifi ed composition, containing certain percentages of vanadium, molybdenum, or other elements, as well as larger amounts of manganese, silicon, and copper than do regular carbon steels.
- Austenetized
- The basic steel structure state in which an alloying is uniformly dissolved into iron.
- Carbon Steels
- Contain varying amounts of carbon and not more than 1.65% of manganese and .60% of copper. There are 3 types of Carbon Steels, Low (.3% or less), Medium (.4-.7%) and High (.8% and up). High carbon is commonly used for knives.
- Corrosion Resistance
- The ability of a material to resist deterioration as a result of a reaction to its environment. Provided by the elements Chromium (Cr), Copper (Cu), Molybdenum (Mo) and Nitrogen (N).
- Critical Temperature
- The temperature at which steel changes its structure to austenite in preparation for hardening.
- Ductility
- The ability of a material to be stretched or drawn, plastically deform appreciably before fracturing. Provided by the element Manganese (Mn).
- Edge Retention
- The ability of a material to resist abrasion and wear. Provided by the elements Carbon (C), Chromium (Cr), Manganese (Mn), Nitrogen (N) and Vanadium (V).
- Exotic Steels
- Are generally accepted as steel, but by definition are not steel. Examples of Exotic Steels include H1, ZDP-189, Talonite and Titanium. There is an old proverb, "There was never a good knife made of bad steel." This statement, just like steel itself, is completely subjective as it relates to knives and knife knuts. We hope this information provides you with a foundation to make your own determinations where steel is concerned.
- Grit
- The physical size of the austenite grains during austenizing. The actual size can vary due to thermal, time and forging considerations.
- Hardenability
- The ability of a steel to be hardened by a heat treating process. Provided by the elements Manganese (Mn), Molybdenum (Mo) and Tungsten (W).
- Hardness
- The resistance of a steel to deformation or penetration analogous to strength.
Heat Treating
Heating and cooling metal to prescribed temperature and the limits for the purpose of changing the properties and behavior of the metal.
High-Strength Low-alloy Steels
Known as HSLA steels are relatively new. They cost less than do regular Alloy Steels because they contain only small amounts of the expensive alloying elements. They have been specially processed, however, to have much more strength than Carbon Steels of the same weight.
Impact Strength
The ability of a material to resist cracking due to a sudden force.
Martensite
A very hard and brittle steel with a distorted body centered tetragon crystal structure.
Precipitation
The separation of a substance that was previously dissolved in another substance.
Quenching
Soaking of steel that has reached a high temperature (above the recrysallization phase) in a medium of air, liquid, oil or water to rapidly cool it. Quenching steel creates martensite.
Rockwell Test
A measurement of steel hardness based on the depth of penetration of a small diamond cone pressed into the steel under a constant load.Stainless
Stainless Steels
Contain a minimum of 12% Chromium. The Chromium provides a much higher degree of rust resistance than Carbon Steels. Various sources site differing minimum amounts of Chromium required to deem a steel as stainless (10-13%). It is important to note, that the amount of Chromium needed can be dependent upon the other elements used in the steel.
Tempering
Reheating to a lower temperature after quenching for the purpose of slightly softening the steel, precipitating carbides, stress relieving.
Tensile Strength
Indicated by the force at which a material breaks due to stretching.
Tool Steels
Contain Tungsten, Molybdenum and other alloying elements that give them extra strength, hardness and resistance to wear.
Toughness
The ability of a material to resist shock or impact.
Yield Strength
The point at which a steel becomes permanently deformed; the point at which the linear relationship of stress to strain changes on a Stress/Strain curve.